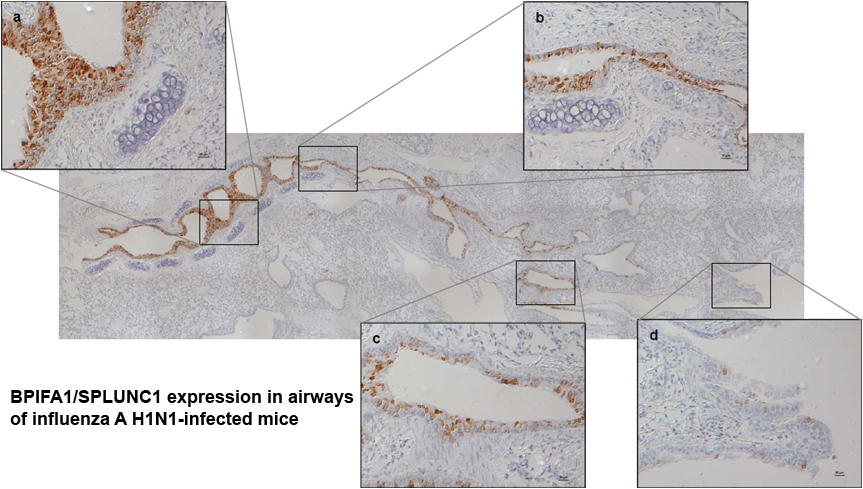
We are investigating the role of BPIFA1 in the host immune response to virus infection using Influenza A virus (IAV) as a model animal virus.
What is known about BPIFA1? The airway epithelium has a fundamental role in the defense against pathogens and as such secretes a number of proteins/peptides that function in innate and adaptive immunity. Bactericidal/permeability-increasing (BPI) fold-containing family A1 (BPIFA1; also called BPIFA1) is a glycoprotein that is highly expressed in the respiratory epithelium and submucosal glands of the upper airways of all air-breathing mammals including mice and humans. Previous studies have demonstrated that BPIFA1 acts as a surfactant, can regulate epithelial sodium channel, ENaC and affects mucociliary clearance in the upper airways. Additionally, it is known to have anti-bacterial roles. For example, Bpifa1 expression is induced after Mycoplasma infection, enhancing IL-8 production and bacterial clearance. BPIFA1 also has a role in defense against Klebsiella pneumoniae, possibly acting through modulation of macrophage function. Little, if any, is known about the anti-viral role of BPIFA1, although our previous work has shown a critical role for BPIFA1 in restricting influenza A virus infection.
We have chosen influenza A virus (IAV) as a model respiratory virus to study BPIFA1 function. IAV is an RNA virus of the Orthomyxovirus genus. IAV strains are endemic and largely asymptomatic in wild birds (avian influenza) but can cause severe disease outbreaks in domestic poultry that are of economic importance. For instance, two recent outbreaks in the EU cost >100 M€. A similar scenario applies to swine influenza. Zoonotic transmission of Influenza A is a major worldwide health concern for persons of all ages. The innate defence to IAV consists of non-specific factors (mucus and collectins) that blunt infection of respiratory epithelial cells. IAV is sensed by infected cells via pattern-recognition receptors (PRRs) that recognise viral RNA. These include toll like receptors (TLRs, specifically TLR7 and TLR3), retinoic acid inducible gene-I (RIG-I) and the NOD-like receptor family pryin domain containing 3 (NLRP3) protein. Signalling by these receptors leads to production of pro-inflammatory cytokines and type I interferons that have antiviral activity. Subsequently, adaptive immunity eliminates infection and provides protection from re-infection. IAV induces virus-specific antibody responses. Antibodies specific for the two surface glycoproteins, HA and NA, are of importance since their presence correlates with protective immunity. In addition, a potent CD8+ T cell response is required for elimination of virus infected cells and for protection from heterologous virus infection as anti-IAV CD8+ T cells often recognise conserved viral epitopes. Despite a lack of natural susceptibility to human influenza viruses, mice provide a well-characterised model with predictive efficacy for the study of the immune response to infection through adapted viruses.
There are vaccines and antiviral drugs to combat influenza. However, due to rapid virus evolution, vaccines need to be re-formulated and re-administered most years and resistance against antiviral drugs is emerging with associated economic cost. For instance, the UK spent £1.24 billion preparing for and responding to the 2009 pandemic. It is therefore important to understand how intrinsic and innate mechanisms modulate influenza virus infection and how these may be used to develop novel therapeutic interventions.
BPIFA1 restricts epithelial infection by influenza A virus
To determine the role of BPIFA1 in host innate and adaptive immunity against (IAV) infection we have developed transgenic mouse models (total and conditional knockout (KO)) that are deficient in BPIFA1 as well as 3D mouse tracheal epithelial cell (mTEC) cultures grown at the air-liquid interface derived from these models. We have shown that BPIFA1 has a significant role in mucosal defence against IAV infection. Mice deficient in BPIFA1 lost more weight after infection, supported a higher viral load and virus spread more rapidly to the peripheral lung, indicative of a defect in the control of infection. Further analysis using mTEC cultures showed that BPIFA1-deficient cells bound more virus particles, displayed increased nuclear import of IAV ribonucleoprotein complexes and supported higher levels of viral replication. These observations were consistent between both X31 (H3N2) and PR8 (H1N1) strains of IAV. Our results identify a critical role for BPIFA1 in the initial phase of infection by inhibiting the binding and entry of IAV into airway epithelial cells. This work has been recently published in Mucosal Immunology.
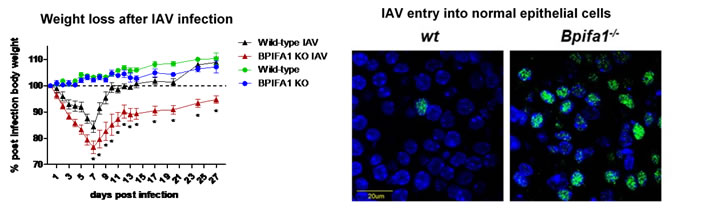
Current studies: BPIFA1 and adaptive immunity
More recently, we have discovered that BPIFA1 is important for the generation of the specific antibody and T cell responses that are generated against influenza A locally in the lung. Current work in the laboratory is focussed on how BPIFA1 can modulate T cell and antibody responses in the respiratory tract, and how this affects protection against influenza A. This work is in collaboration with Colin Bingle (University of Sheffield) and is funded by a BBSRC project grant and an MRC studentship
"Mucosal Immunology" front cover image taken from our publication


Relevant Group Publications
2018
2016
2015
2012
Posters
Posters relating to this work presented at international meetings have been submitted to Faculty of 1000 posters and can be found using the links below
Alterations in SPLUNC1 expression in rodent models of respiratory virus infection - presented at the Biochemical Society 2011 - Proteins with a BPI/LBP/Plunc-like Domain: Revisiting the Old and Characterizing the New meeting, 5 - 7 Jan 2011

