This project investigates the role of non-cannonical autophagy or LC3 associated phagocytosis (LAP) in the host response to virus infection using Influenza A virus (IAV) as a model animal virus to determine the role played by LAP during an acute, lytic viral infection in epithelial cells of the respiratory tract and lung. It is being performed in collaboration with Tom Wileman (University of East Anglia/Quadram Institute) and is funded by the BBSRC.
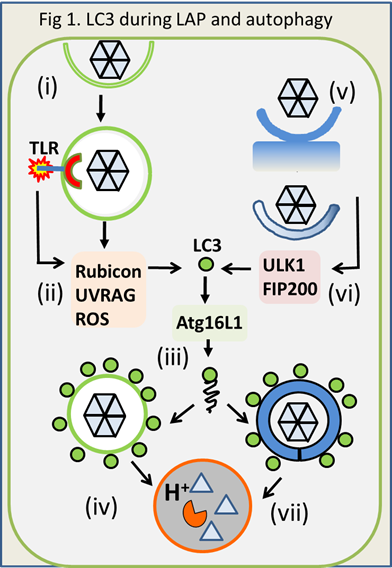 Autophagy and LC3 associated phagocytosis (LAP). LAP is a recently characterised phagocytic pathway that plays important roles during innate immunity to pathogens. Phagosomes containing pathogens, apoptotic cells and apoptotic cells infected with viruses, are sensed as ‘foreign or damaged’ (Fig1 i,ii), and recruitment of the autophagy protein LC3 marks them for fusion with lysosomes (iii,iv). Degradation in lysosomes kills pathogens, and delivery to class II MHC compartments and cross presentation on MHC class I, enhances presentation of microbial components to the acquired immune system. Recognition of damaged endosomes and lysosomes by LAP is not restricted to myeloid cells, and is likely to occur in many cell types. Autophagy and LAP are related pathways but autophagy differs from LAP because pathogens targeted for autophagy are in the cytoplasm ‘endogenous’ (Fig 1 v) and engulfed by autophagosomes generated from cellular membrane compartments (Fig 1 vi), rather than in endosomes ‘exogenous’ entering the cell from outside.
Autophagy and LC3 associated phagocytosis (LAP). LAP is a recently characterised phagocytic pathway that plays important roles during innate immunity to pathogens. Phagosomes containing pathogens, apoptotic cells and apoptotic cells infected with viruses, are sensed as ‘foreign or damaged’ (Fig1 i,ii), and recruitment of the autophagy protein LC3 marks them for fusion with lysosomes (iii,iv). Degradation in lysosomes kills pathogens, and delivery to class II MHC compartments and cross presentation on MHC class I, enhances presentation of microbial components to the acquired immune system. Recognition of damaged endosomes and lysosomes by LAP is not restricted to myeloid cells, and is likely to occur in many cell types. Autophagy and LAP are related pathways but autophagy differs from LAP because pathogens targeted for autophagy are in the cytoplasm ‘endogenous’ (Fig 1 v) and engulfed by autophagosomes generated from cellular membrane compartments (Fig 1 vi), rather than in endosomes ‘exogenous’ entering the cell from outside.
LAP shares many components with the autophagy pathway but differs in signalling events upstream of LC3 recruitment to membranes. LAP is activated during phagocytosis by pattern recognition receptors (ii), and requires Rubicon, UVRAG and NOX2/ROS (ii) to recruit LC3 (1). Autophagy requires the ULK1/FIP200 complex (vi) for recruitment of LC3, but the ULK1 complex is not required for LAP.
Dissecting autophagy and LAP. Autophagy and LAP play important roles in controlling infections, but the precise role played by each pathway ‘in vivo’ has been very difficult to dissect. Autophagy and LAP share much of the machinery needed for recruitment of LC3 to membranes and this means that tissue-specific knock out of essential autophagy proteins, such as Atg5, 7 and 16, causes loss of both pathways.
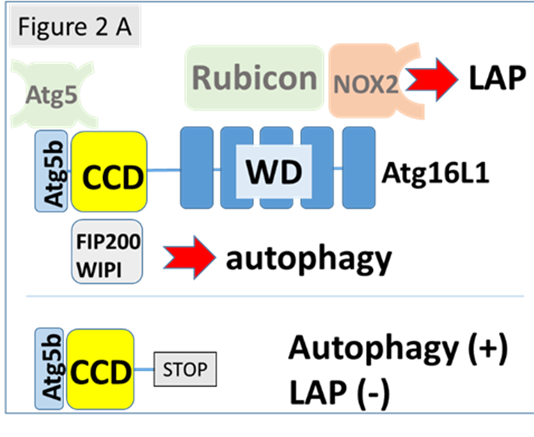 This project is based on mice generated at UEA that are deficient in LAP, but have normal autophagy. These mice (E230) lack the WD domain of ATG16L1 (Fig 2A) and are entirely normal apart from the deficiency in LAP. (Fletcher et al. (2018) EMBO J 37:e97840). This allows us to focus specifically on the role played by LAP in the control of viral infection, without concern about the bystander effects resulting from loss of autophagy and consequent changes in tissue homeostasis, lymphoid differentiation and lowered inflammatory threshold.
This project is based on mice generated at UEA that are deficient in LAP, but have normal autophagy. These mice (E230) lack the WD domain of ATG16L1 (Fig 2A) and are entirely normal apart from the deficiency in LAP. (Fletcher et al. (2018) EMBO J 37:e97840). This allows us to focus specifically on the role played by LAP in the control of viral infection, without concern about the bystander effects resulting from loss of autophagy and consequent changes in tissue homeostasis, lymphoid differentiation and lowered inflammatory threshold.
This project will use E230 LAP-deficient mice to characterise the role played by LAP during influenza A infection and also the relative contributions of myeloid and epithelial cells to this process

Relevant Publications
2020

