In this project we are developing a new approach to respiratory virus vaccination by exploiting a novel Bacteroides thetaiotaomicron (Bt) based mucosal antigen delivery platform. This is being developed as novel means of delivering mucosal vaccination for viral disease including influenza and pest de petite ruminants (PPR)
Vaccination is an efficient and cost-effective form of infectious disease prevention that can lead to their global eradication, as seen for smallpox (1980) and rinderpest (2011). Veterinary vaccines are routinely used to control infections, enhance animal welfare and sustainably improve livestock production to meet global food security challenges. However, there is an urgent and growing need for the development of new and improved vaccines to further reduce the global burden of infectious disease morbidity and mortality. Vaccines to combat zoonoses are a particularly urgent priority as 60% or more pathogens with the potential to harm humans, originate in animals. Vaccines delivered systemically do not always produce effective immunity at mucosal surfaces. Also, despite the majority of infectious agents entering the body via mucosal sites, there are few licensed mucosal vaccines.
A major challenge in mucosal vaccine development is the delivery of intact, biologically active vaccine Ag to mucosa-associated lymphoid tissues. To address this, we are using naturally-generated nanoparticles, in the form of OMVs produced by the prominent Gram-negative gut bacterium Bt, to deliver IAV vaccine Ag to the respiratory tract where they will induce protective mucosal and systemic immune responses. Nanoscale OMVs are highly stable proteoliposomes that contain immunogenic components derived from the bacterial outer membrane and periplasm, and can target APCs leading to T and B cell immunity. OMVs from Neisseria meningitides and Vibrio cholera are used in licensed vaccine formulations of proven safety and effectiveness and N. meningitides OMVs successfully control serogroup B meningococcal disease outbreaks.
OMV based vaccines have significant advantages over conventional vaccines as they: are non-replicating; can be delivered orally and intra-nasally overcoming the need for needles; target mucosal sites; have an established safety record; elicit innate and Ag-specific adaptive immune responses; possess self-adjuvant properties; are relatively cheap and straightforward to produce; are compatible with rapid re-formulation and vaccine production; can be delivered outside a formal clinic setting; have no cold-chain requirement. This makes OMVs an attractive option for vaccination, especially in resource-limited and remote rural areas. Limitations of current, pathogen-derived, OMV vaccines include: the potential for unintended pathology due to associated toxins; low expression levels of protective Ag; variable efficacy depending on source and formulation; possible need for exogenous adjuvants; potential for incomplete protection due to strain variation. We can overcome these limitations using non-pathogenic OMVs from commensal bacteria and genetic engineering to improve their vaccine application.
We have found that Bt-OMVs access nasal associated lymphoid tissue (NALT) and other respiratory lymphoid tissues to initiate mucosal (IgA) immune responses. Importantly, we have demonstrated that intra-nasal delivery of Bt-OMVs containing the Salmonella vaccine Ag, SseB induced both mucosal (IgA in BAL) and systemic (IgG in the serum) Ag-specific antibody (Ab) responses with no pathological consequences.
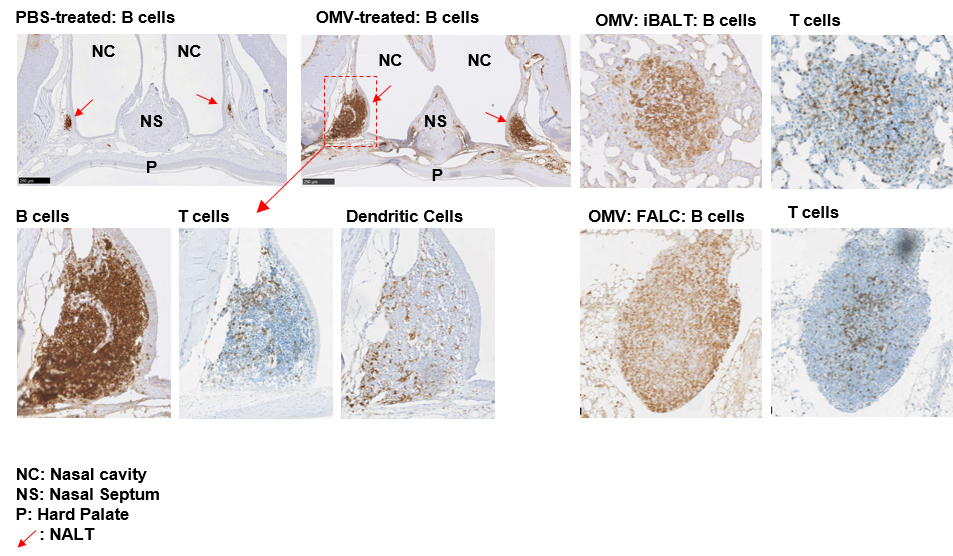
Sections of nasal cavities showing lymphoid aggregates. In mice, intra-nasally administered Bt-OMVs promote the development of large lymphoid aggregates and follicles with germinal centre formation in the nasal cavity (NC)-associated NALT (arrows) and lung-associated iBALT identified by anti-CD45R (B cell) Ab staining. T cells (anti-CD3) and MΦ/DCs (anti-Iba-1) were present in the NALT (arrowed boxed region) with B and T cell aggregates also seen in the fat-associated lymphoid clusters (FALC) of lung adipose tissue. NS = nasal septum.
OMVs as a novel vaccine for influenza
Influenza is an economically significant zoonotic disease; new strains spread rapidly causing catastrophic pandemics. Due to rapid virus evolution, available vaccines/ antiviral drugs must be re-formulated and re-administered in most years. Drug resistance is emerging with associated economic costs. In the wake of the influenza H5N1 pandemic, millions of poultry were killed; over 50 million domestic birds in Vietnam alone. A 2005 Food and Agricultural Organisation (FAO) report estimated economic losses of $10 billion in South East Asia and the UK spent £1.24 billion preparing for and responding to the 2009 pandemic. Also, 40-50% of the circulating strains of flu in the current 2017/2018 influenza outbreak do not match those in the 2017/18 vaccine (Health Protection Scotland, Respiratory Report 7th Jan. 2018). There is, therefore, an urgent need for ‘universal’ IAV therapeutics, independent of the vagaries of continuous viral mutations.
We are using influenza A virus (IAV) infection of mice as a model system to test the usefulness of Bt OMVs in influenza vaccination. Bt have been engineered to express (IAV) vaccine Ag in outer-membrane vesicles (OMVs) and are being tested for efficacy by delivery via the respiratory route to the mucosal immune system where it will induce local and systemic immunity.
This project is being performed in collaboration with Simon Carding (Quadram Institute Bioscience).

- Carvalho, A.L., Fonseca, S., Miquel-Clopes, A., Kok, K-S, Wegmann, U., Gil-Cardoso, K., Bentley, E., Kipar, A., Stentz, R., Stewart, J.P., Carding, S.R. (2019). Bioengineering human gut commensal bacteria derived outer membrane vesicles for the delivery of biologics to the gastrointestinal and respiratory tract. Journal of Extracellular Vesicles. 8:1, DOI: 10.1080/20013078.2019.1632100 [PDF]

- Miquel-Clopés, A., Bentley, E.G., Stewart, J.P., Carding, S.R. (2019). Mucosal vaccines and technology. Clinical and Experimental Immunology,196 205-214. https://doi.org/10.1111/cei.13285 [PDF]

Relevant Group Publications
2019

