What is Malignant Catarrhal Fever (MCF)?
Malignant catarrhal fever (MCF) is a severe, usually fatal lymphoproliferative and inflammatory syndrome of domestic cattle, pigs, deer and certain other susceptible ruminants such as bison. The disease is mainly caused by either of two closely-related bovid gammaherpesviruses (γHVs), alcelaphine herpesvirus 1 (AlHV-1) and ovine herpesvirus 2 (OvHV-2), that persist sub-clinically in their natural host. These viruses are highly related in biological properties and sequence to each other and to porcine lymphotropic herpesvirus 1 (PLHV-1) that causes post-transplant lymphoproliferative disease in pigs.
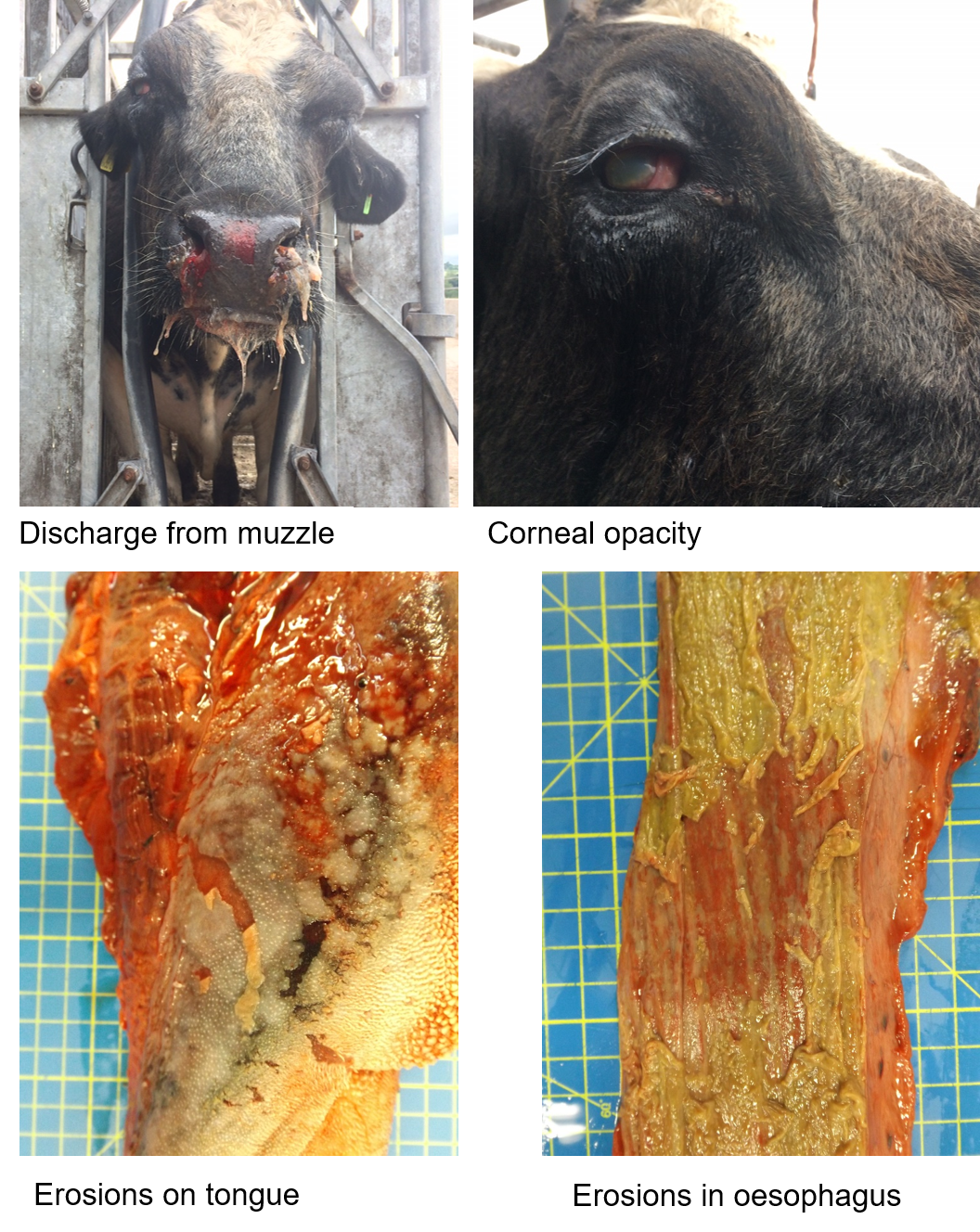
Why Study MCF?
MCF is normally sporadic in the UK but periodically outbreaks occur where losses on individual (and adjacent) premises can be substantial. Annually, 100-130 cases are diagnosed and reported (AHVLA, Veterinary Investigation Surveillance Reports). This figure is thought to be a large underestimate as only one or a few animals are tested in each outbreak as other deaths in the herd are assumed to be MCF and are not recorded. The outcome is usually fatal. It is treated as a serious problem by DEFRA/AHVLA and stakeholders (farmers and veterinarians). Aside from sporadic outbreaks in domestic cattle, MCF is the most important virus disease of farmed deer and has recently been reported in pigs. MCF is also currently a disease of great concern in Indonesia, affecting Bali cattle and in the U.S.A. where bison are particularly susceptible .
AlHV-1 naturally infects wildebeest (Connochaetes spp) and is the source of wildebeest-associated MCF in Africa. OvHV-2 is endemic in domestic sheep (Ovis aries), which act as a reservoir of infection for the other main form of the disease, sheep-associated MCF.
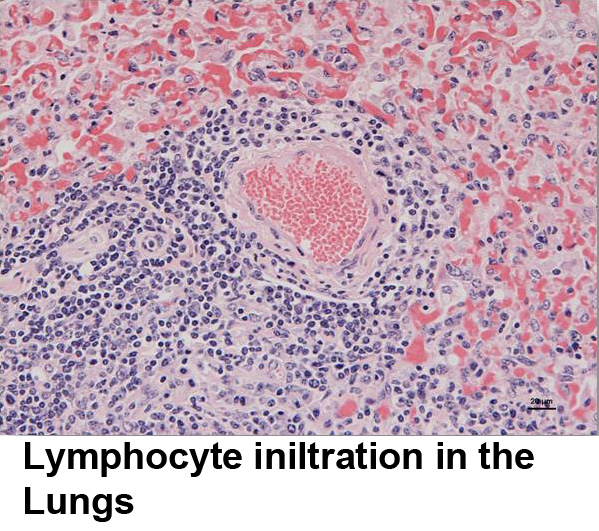
In the reservoir species, sheep, OvHV-2 DNA has been found by PCR in B cells in the bloodstream, lymph nodes and the respiratory, alimentary, and urogenital tracts. OvHV-2 DNA has also been detected in nasal and ejaculatory secretions suggesting possible respiratory and sexual transmission mechanisms. Moreover, cattle have been experimentally infected with nasal secretions from infected sheep showing that respiratory transmission is likely. In contrast, in SA-MCF-affected ruminants, virus DNA is usually detected by PCR in lymph nodes and spleens and has been observed by in situ hybridization in hyperplastic T cells in brain lesions. Thus, to enable the study of the interaction of OvHV-2 with host cells, T-lymphoblastoid cell lines with the morphology of large granular lymphocytes (LGLs) have be established in culture from the tissues of MCF-affected animals . These T cell lines contain OvHV-2 DNA and antigen and can transmit MCF experimentally to rabbits and hamsters which are used as animal models. OvHV-2-positive LGLs generally have a T cell phenotype, are constitutively and indiscriminately (non-MHC-restricted) cytotoxic and produce a range of cytokines, but not IL-2. Our current hypothesis is that MCF is due to indiscriminate tissue damage caused by dysregulated cytotoxic T cells generated as a consequence of infection. The LGL T cells in culture represent the virus-infected cells in vivo and are invaluable for virus-cell interaction studies in MCF.
AlHV-1 has been isolated, will productively infect epithelial cell lines in culture and has been completely sequenced. In contrast, research on OvHV-2 has lagged behind due to the lack of a productive tissue culture system and reagents. However, the Stewart group cloned the OvHV-2 genome from an affected cow as a set of overlapping cosmids and have sequenced the entire genome in collaboration with Mathias Ackermann (University of Zurich) and Hugh Reid (Moredun Research Institue). This is extremely similar to a sequence that was subsequently derived from the nasal secretions of sheep by Hong Li and colleagues (WSU).
Current work includes using molecular techniques to define the function of individual AlHV-1 and OvHV-2 genes in the pathogenesis of MCF and defining the pathogenesis of OvHV-2 in sheep which are reservoir species.
For further information on MCF read this review

Relevant Group Publications
- Dry, I., Todd, H., Deane, D., Percival, A., Mclean, K., Inglis, N.F., Manson, E.D.T., Haig, D.M., Nayuni, S., Hutt-Fletcher, L.M., Grant, D.M., Bartley, K., Stewart, J.P. and Russell, G.C. (2016). Alcelaphine herpesvirus 1 glycoprotein B: recombinant expression and antibody recognition. Archives of Virology. 161. 619-619. .[PDF]
 doi:10.1007/s00705-015-2701-y
doi:10.1007/s00705-015-2701-y - Amin, DM., Stewart, JP, Kipar, A. (2016). Distribution of Ovine Herpesvirus-2 Transcript and Protein in Naturally Infected Sheep. Journal of Comparative Pathology, 154. 119.
- Russell, G.C., Scholes, S.F., Twomey, D.F., Courtenay, A.E., Grant, D.M., Lamond, B., Norris, D., Willoughby, K., Haig, D.M. and Stewart, J.P. (2014) Analysis of the genetic diversity of ovine herpesvirus 2 in samples from livestock with Malignant Catarrhal Fever. Veterinary Microbiology, 172. 63-71. [Full Text on Publisher Website] [PDF]

- Russell, G.C., Deane, D., Percival, A., Todd, H., Haig, D.M., and Stewart J.P. (2013). A novel spliced gene in alcelaphine herpesvirus 1 encodes a glycoprotein which is secreted in vitro. Journal of General Virology. 94, 2515-2523. DOI:10.1099/vir.0.055673-0 [Full Text on Publisher Website][PDF]

2016
2014
2013
2009
- Russell, G.C., Stewart, J.P. and Haig, D.M. (2009). Malignant Catarrhal Fever. The Veterinary Journal. 179, 324-335. (Review). [Full Text on Publisher Website] [PDF]
 *Recipient of The Veterinary Journal review prize 2009
*Recipient of The Veterinary Journal review prize 2009
- Meier-Trummer, C., Tobler, K., Hilbe, M., Stewart, J.P., Hart, J., Campbell, I., Haig, D.M., Glauser, D., Ehrensperger, F., Ackermann, M. (2009). Ovine herpesvirus-2 structural proteins in epithelial cells and M-cells of the appendix in rabbits with malignant catarrhal fever. Veterinary Microbiology. 137, 235-242. [Full Text on Publisher Website] [PDF]

- Jayawardane, G., Russell, G.C., Thompson, J., Deane, D., Cox, H., Gatherer, D., Ackermann, M., Haig, D.M., and Stewart J.P. (2008). A captured viral interleukin 10 gene with cellular exon Structure. Journal of General Virology. 89, 2447-2455. [Open Access Full Text] [PDF]

- Dry, I., Haig, D.M., Inglis, N.F., Imrie, L., Stewart, J.P. and Russell, G.C. (2008). Proteomic analysis of pathogenic and attenuated Alcelaphine herpesvirus-1. Journal of Virology. 82, 5390-5397. [Open Access Full Text] [PDF]

- Anderson, I.E., Deane, D., Swa, S., Thomson, J., Campbell, I., Buxton, D., Wei, X-Q., Stewart, J.P, Russell, G. and Haig, D.M. (2008). Production and utilisation of interleukin-15 in malignant catarrhal fever. Journal of Comparative Pathology, 138, 131-144. [Full Text on Publisher Website] [PDF]

- Hart, J., Ackermann, M., Jayawardane, G., Russell, G., Haig, D.M., Reid, H. and Stewart, J.P. (2007). Complete sequence and analysis of the ovine herpesvirus 2 genome. Journal of General Virology. 88, 28-39. [Open Access Full Text] [PDF]

- Taus, N.S., Herndon, D.R., Traul, D.L., Stewart, J.P., Ackermann, M., Li, H., Knowles, D.P., Lewis, G.S. and Brayton, K.A. (2007). Comparison of ovine herpesvirus 2 genomes isolated from domestic sheep (Ovis aries) and a clinically affected cow (Bos bovis). Journal of General Virology. 88, 40-45. [Open Access Full Text] [PDF]

- Thonur, L., Russell, G., Stewart J.P. and Haig, D. (2006). Differential transcription of ovine herpesvirus 2 genes in lymphocytes from reservoir and susceptible species. Virus Genes. 32, 27-35. [Full Text on Publisher Website] [PDF]

- Wright, H., Stewart, J.P., Ireri, R.G., Campbell, I., Pow, I., Reid, H.W. and Haig, D.M. (2003). Genome Rearrangements associated with Loss of Pathogenicity of the g-Herpesvirus Alcelaphine Herpesvirus –1. Research in Veterinary Science. 75, 163-168. [Full Text on Publisher Website] [PDF]

- Rosbottom, J., Dalziel, R.G., Reid, H.W. and Stewart, J.P. (2002). Ovine herpesvirus 2 lytic cycle replication and capsid production. Journal of General Virology. 83, 2999-3002. [Open Access Full Text] [PDF]

2008
2007
2006
2003
2002

